New pharmaceuticals are becoming increasingly effective and giving patients new hope. In this context, highly active ingredients are growing in importance. Safe containment solutions from Fette Compacting avoid risks to personnel and the environment.
With rapid growth in the market segment for highly active ingredients, Containment concepts are increasingly becoming the focus of tablet production. Containment uses technical and organizational measures to ensure that operating personnel and the environment are reliably protected from active and highly active ingredients and that there is no cross-contamination with other substances.
A sugar cube in the Elbphilharmonie
Due to the significant health risks, active and highly active ingredients are subject to strict regulatory requirements. Standards such as Occupational Exposure Bands, Occupational Exposure Limits, and Permitted Daily Exposure specify the maximum permissible concentration of active ingredients in the air. For highly active substances, known as HPAPIs (High Potency Active Pharmaceutical Ingredients), these limits are very low – often in the range of a few micrograms, in some cases even in the nanogram range per cubic meter of air.
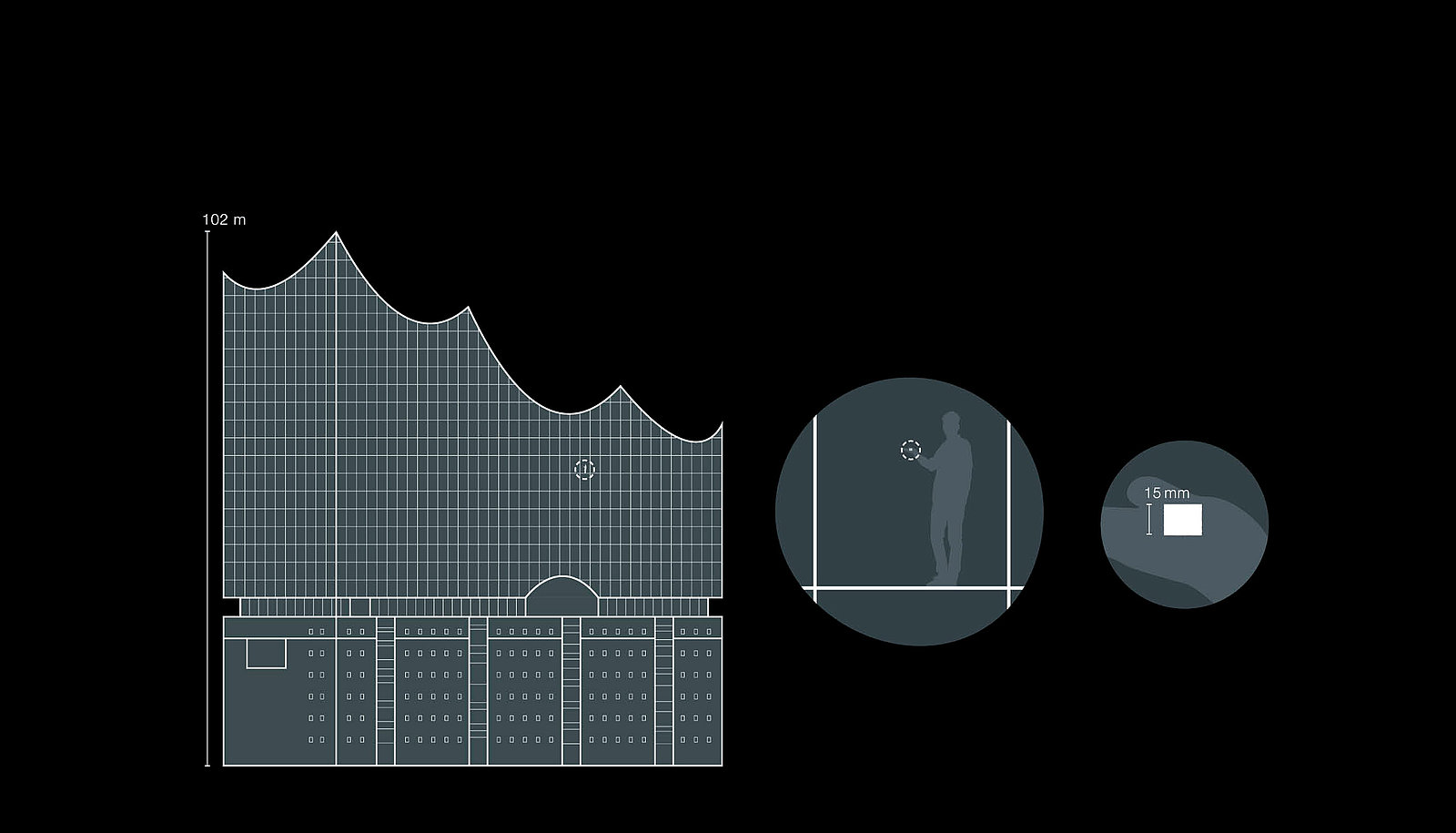 Apart from assigning the limit values to the substance systems, it is also hard to imagine the magnitude of the concentrations.
Apart from assigning the limit values to the substance systems, it is also hard to imagine the magnitude of the concentrations.
A vivid comparison illustrates the extreme requirements: if a single sugar cube were to be ground up and spread throughout the Elbphilharmonie concert hall in Hamburg, this would correspond to an indoor air concentration of 5.5 micrograms per cubic meter. This is a limit value that containment systems must comply with on a daily basis and in some cases even fall significantly below.
From dust-tight to high-secure
Modern containment solutions are as diverse as the requirements themselves. The range extends from dust-tight basic equipment to complex high-security systems. Many tablet presses from Fette Compacting already operate in a dust-tight manner and under constant negative pressure in their standard version.
For increased safety requirements, Fette Compacting offers extended containment packages for tablet presses and other devices such as isolators or process equipment. These include:
Hermetically sealed window flaps
Permanently installed glove ports for manual interventions without interrupting containment
Rapid transfer ports (RTP) for the safe transfer of components
Manual suction devices for manual pre-cleaning
Permanent negative and positive pressure monitoring

 Hermetically sealed window flaps protect the operating personnel.
Hermetically sealed window flaps protect the operating personnel.
 Operating personnel use glove ports to perform manual interventions without interrupting containment.
Operating personnel use glove ports to perform manual interventions without interrupting containment.
 The Rapid Transfer Port allows operators to safely insert and replace tableting tools into the tablet press.
The Rapid Transfer Port allows operators to safely insert and replace tableting tools into the tablet press.
Comprehensive services and consulting
For the most demanding scenarios in the high-containment area, Fette Compacting relies on isolator technology with integrated process technology. This completely encapsulates the production area and creates a hermetically sealed working environment. These high-containment systems also offer wash-in-place technologies, WiP for short, which enable automatic and safe cleaning. Thanks to modular extensions, the systems can be ideally adapted to specific production requirements.
 2090i WiP with isolator technology for process equipment and air management systems
2090i WiP with isolator technology for process equipment and air management systems
Fette Compacting has been using Containment Guard since 2017 to reliably validate protective measures. This process systematically measures the containment performance of the systems and documents the safety standards achieved. This ensures that the systems are optimally tailored to the respective requirements.
In addition to technology, Fette Compacting offers comprehensive services: individual consultations, targeted training for users, and ongoing technical support. Fette Compacting also provides validation support to meet regulatory requirements and ensure the best possible safety for personnel and products.
Containment: From necessity to strategic advantage
The future of containment lies in comprehensive, integrated approaches. Instead of isolated individual solutions, Fette Compacting develops holistic strategies that accompany the entire process from the laboratory to large-scale production. An essential part of this approach is the seamless integration of containment into continuous manufacturing, which makes the production of pharmaceutical products even more efficient and safer.
Innovative technologies such as washable air management, VR-supported training for operating personnel, and advancing digitalization are promoting this development and supporting the implementation of holistic solutions. This approach transforms containment from a technical necessity into a strategic advantage: Pharmaceutical companies can combine the highest safety standards with economic efficiency and optimally prepare themselves for the challenges of the growing HPAPI market.